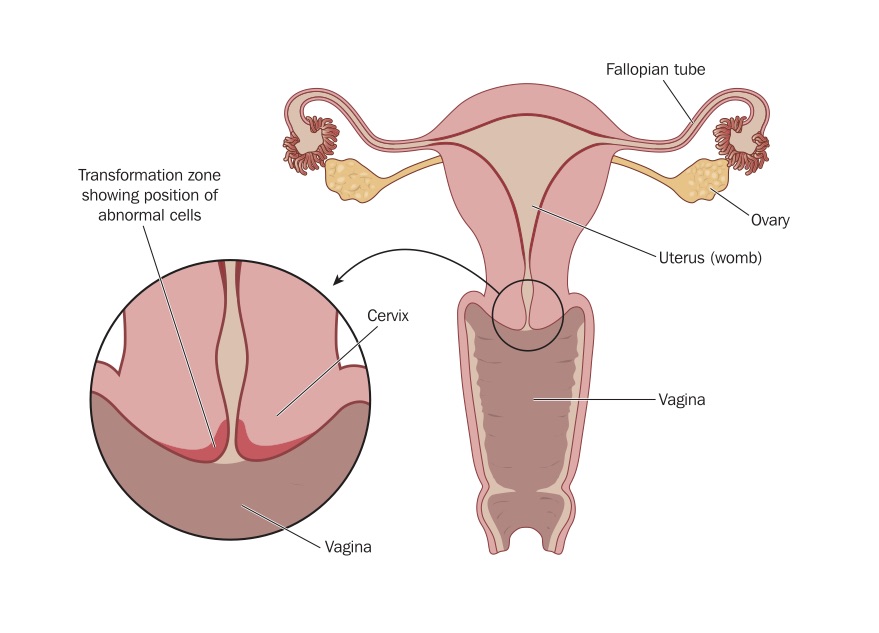
Das Zervixkarzinom lateinisch Carcinoma cervicis uteri auch Kollumkarzinom von lateinisch Collum Hals oder Gebärmutterhalskrebs genannt ist ein bösartiger maligner. Das Zervixkarzinom ist eine maligne Entartung des Gebärmutterhalses Das Zervixkarzinom ist mit einer Inzidenz von jährlich weltweit circa 500000 Betroffenen die. Dictcc Übersetzungen für cervical cancer im Englisch-Deutsch-Wörterbuch mit echten. Cervical cancer is a cancer arising from the cervix 2 It is due to the abnormal growth of cells that have the ability to invade or spread to other parts of the body. Viele übersetzte Beispielsätze mit cervical cancer Deutsch-Englisch Wörterbuch und Suchmaschine für Millionen von Deutsch-Übersetzungen..
What Should I Know About Screening The HPV test looks for the virus human papillomavirus that can cause cell changes on the cervix. Where to get screened for cervical cancer your state or local health department the National Breast and Cervical Cancer Early Detection. CDCs National Breast and Cervical Cancer Early Detection Program provides low-cost breast and cervical cancer screenings. Routine cervical cancer screening is very effective for preventing cervical cancer and deaths from the disease. In both tests cells are taken from the cervix and sent to a lab for testing A Pap test looks for abnormal cells An HPV test looks for infection with the types of..
January is Cervical Cancer Awareness Month an ideal chance for WHO and partners to raise awareness of cervical cancer and vaccination against human. Cervical Cancer Awareness Month is an important opportunity to share evidence-based information promote vaccination against human. January is Cervical Cancer Awareness Month and this year Cervivor is launching a transformative campaign with a bold vision. Cervical Cancer Awareness Month 2024 focuses on educating women about their bodies and reproductive health. Each country should meet the 907090 targets by 2030 to get on the path towards eliminating cervical cancer by the end of this century..
FDA also granted regular approval to pembrolizumab as a single agent for patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy whose. On January 12 2024 the Food and Drug Administration approved pembrolizumab Keytruda Merck with chemoradiotherapy CRT for patients with FIGO 2014 Stage III-IVA cervical. The recommended pembrolizumab dose for treatment of cervical cancer is 200 mg every 3 weeks View full prescribing information for Keytruda FDA granted this application priority review. On October 13 2021 the Food and Drug Administration FDA approved pembrolizumab Keytruda in combination with chemotherapy to treat advanced. Based on the KEYNOTE-158 results the US Food and Drug Administration FDA approved pembrolizumab in this particular unmet needs population of advanced PD-L1..

Komentar